Home > News > News detail
Proposal Submission
Please complete a proposal for each category you are nominated in. All proposals must be submitted to ewen.ward@hansonwade.com no later than Friday, September 27th in order to be entered into the judging process.
Award Category:
Group/Individuals involved:
In no more than 500 words please summarize why you think you are best placed to win the category:
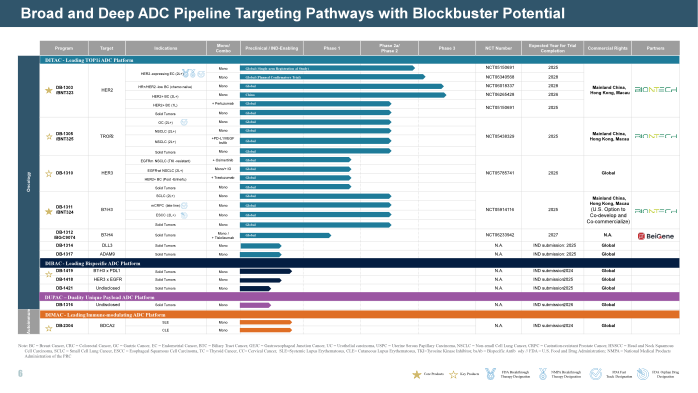
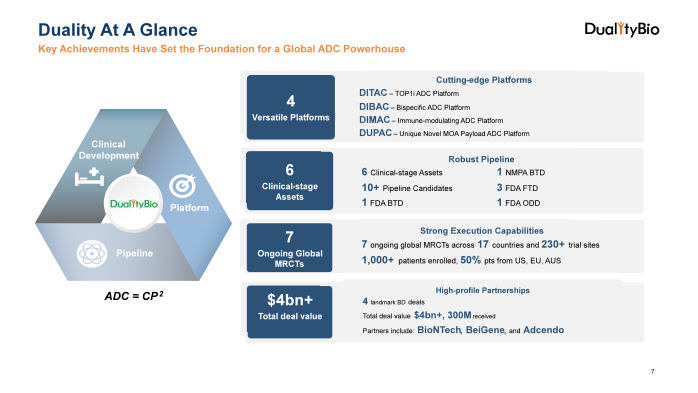
Founded in 2019, Duality Biologics is rapidly emerging as a leading global innovator in antibody-drug conjugate, with a robust pipeline of 12 in-house discovered ADC candidates, including six in global clinical stages, with three receiving FDA Fast Track Designation (FTD). The leading asset, DB-1303, has also been granted Breakthrough Therapy Designation (BTD) by the FDA and NMPA.
Leveraging our experienced R&D team, we have established four cutting-edge ADC technology platforms: DITAC, DIBAC, DIMAC, and DUPAC. validated by its pipeline assets and recognized by global partners
In 2024, Duality is conducting large-scale trials including seven multi-regional clinical trials (MRCTs) spanning 17 countries and over 1,000 enrolled patients, expediting its therapies toward FDA market approval.
In 2024, DB-1303 (HER2 ADC) is advancing in late-stage clinical trials, with a planned BLA submission to the FDA for endometrial cancer by 2025. Our Trop2 ADC (DB-1305) showed promising efficacy in ovarian cancer and potential to be a backbone for combination therapy in NSCLC, TNBC, OC, and CC. We are moving DB-1310 (HER3 ADC) combo therapy to the front lines to cover a broader patient population resistant to EGFR TKI. DB-1311 (B7-H3 ADC) demonstrated robust efficacy and safety in SCLC, CRPC and NSCLC, will registrational trials planned in 2025. in Sep 2024, our Bispecific ADC DB-1419 (PD-L1/B7H3 ADC) entered global FIH clinical with FDA IND approval.
reflecting our visionary foresight into the future landscape of ADC, we are building DUPAC (Duality Unique Payload Antibody Conjugate) to develop linker-payload complexes with novel mechanisms of action beyond traditional cytotoxic agents to combat growing drug resistance to TOP1i-based ADC and hard-to-treat tumors.
Duality Bio is extending the utility of ADCs beyond oncology, with DB-2304, a first-in-class BDCA2 ADC, targeting systemic lupus erythematosus (SLE) and cutaneous lupus erythematosus (CLE). This novel approach to autoimmune diseases opens new avenues for the application of ADC technology in chronic and inflammatory conditions, reinforcing Duality’s innovative edge
Duality’s partnerships with industry leaders like BioNTech, BeiGene and Adcendo have generated over $4.0 billion in deal value, with more than $300M upfront received. The recent filing of its A1 application to the HKEX highlights the company's growth and global ambitions
Any additional figures or statistics you would like to include that support the proposal above:
Please detail any supplementary piece of material you’d like to include as part of your proposal (1 piece maximum per proposal):
Please check the attached short introduction of Duality
Thank you for submitting your World ADC Awards proposal. This will now be reviewed by the Judging Panel.
Duality Biologics Orally Presents Phase 1 Healthy Volunteer Data for First-in-Class BDCA2-Targeted ADC DB-2304 at Autumn Immunology Conference 2025
First Patient Dosed in Phase I/IIa Study of DualityBio’s BDCA2-Targeting ADC DB-2304 for Systemic Lupus Erythematosus
Fast Track Designation Granted for AVZO-1418/DB-1418, a Potential Best-in-Class EGFR/HER3 Bispecific Antibody-Drug Conjugate, for the Treatment of Patients with EGFR-Mutated TKI-Pretreated NSCLC
For more information, please
follow the official WeChat public
