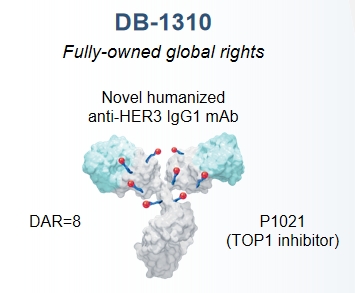
Main highlights of drug design
· Binding novel epitope on domain I of HER3. Partially block HER2/HER3 and NRG/HER3 interaction
· Higher affinity than U3-1402
· High internalization capability
Home > Research > Product pipeline > DB-1310
· Overexpression in multiple tumor types and associated with drug resistance
· Pathway synergies with HER2 and EGFR dimerization
· Subsequent therapy amid growing HER2 ADC r/r population

Main highlights of drug design
· Binding novel epitope on domain I of HER3. Partially block HER2/HER3 and NRG/HER3 interaction
· Higher affinity than U3-1402
· High internalization capability