Main highlights of drug design
First-wave DITAC-empowered Asset under Global Clinical Development
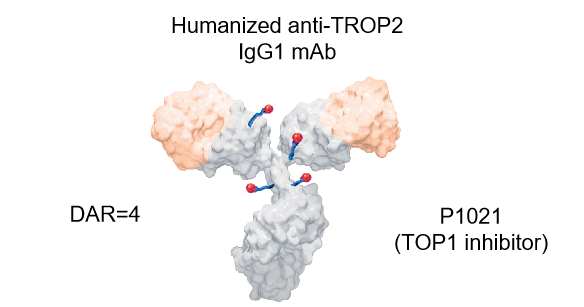
· Well-positioned to address underserved needs in OC, NSCLC and TNBC
· Combination potential as backbone therapy in multiple solid tumors (NSCLC, TNBC, OC, CC)

Main highlights of drug design
First-wave DITAC-empowered Asset under Global Clinical Development