Main highlights of drug design
・ Potent tumor killing while reducing off-target toxicities
・ High selectivity by targeting the 4IgB7-H3 isoform (predominantly found on B7-H3-overexpressing tumor cells)
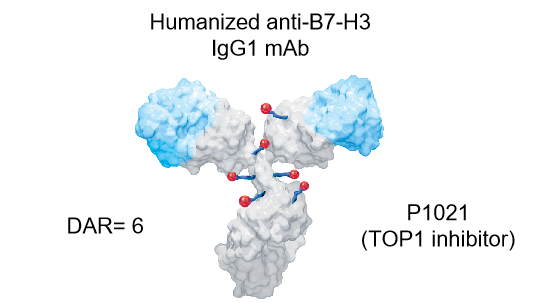
・ A clinically advanced B7-H3 ADC candidate with global market potential
・ Combination potential as frontline treatment for prevalent cancers
・ Opt-in rights to co-develop and co-commercialize in the U.S.

Main highlights of drug design
・ Potent tumor killing while reducing off-target toxicities
・ High selectivity by targeting the 4IgB7-H3 isoform (predominantly found on B7-H3-overexpressing tumor cells)